SCIENCE
The problem with liquid liposomes, (both injectable and oral delivery), is that they degrade quite quickly and have a short half-life. They are also more likely to break down in the stomach although it is not to say that they are ineffective, but less stable. Liquid liposomes tend to show low-solubility and sometimes leakage of the encapsulated molecules.
There have been some innovative methods invented to handle these problems and of these, so-called prolipsomes has been the most promising. Development of these dry liposomes began in the 1980’s for use in the pharmaceutical world.
LipoCellTech™ liposomes are in fact proliposomes
The stability of prolipsomes is far superior to regular liquid liposomes. They are a dry, free-flowing granular material (powder), that immediately forms a liposomal dispersion on contact with water or a biological fluid within the body. When the dry powders are hydrated with water or body fluids followed by gentle mixing, (as in the stomach), the contents rapidly disperse to give a liposomal suspension in the aqueous solution. In fact, THE LIPOSOMES FORMED, ONCE RECONSTRUCTED IN THE BODY, ARE SIMILAR TO CONVENTIONAL LIPOSOMES AND MORE STABLE AND UNIFORM IN SIZE.
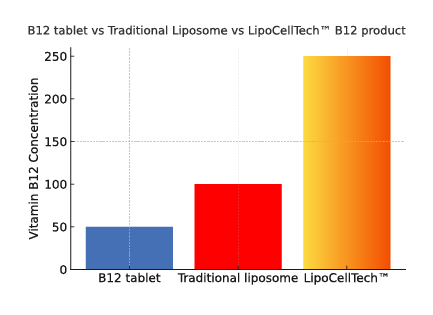
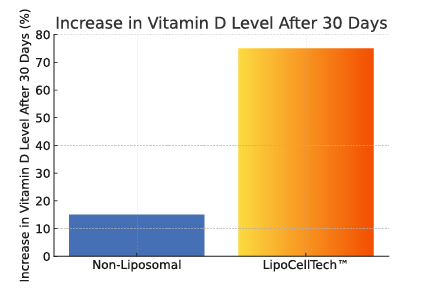
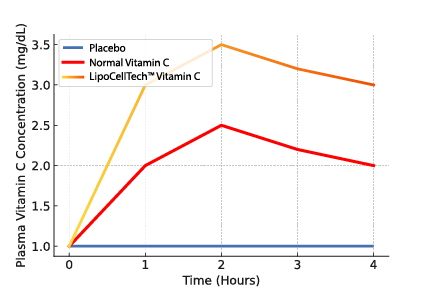
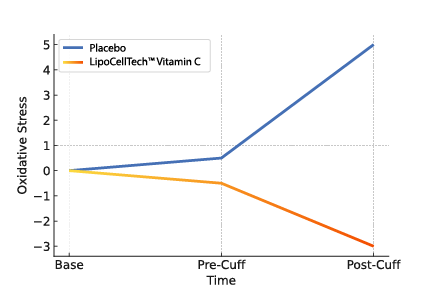
To summarize; Our dry liposomes (proliposomes), are more stable, thus have a longer half-life. This way ensuring that a lot more of the ingredients in the liposomes reach the cells intact. 1, 2.
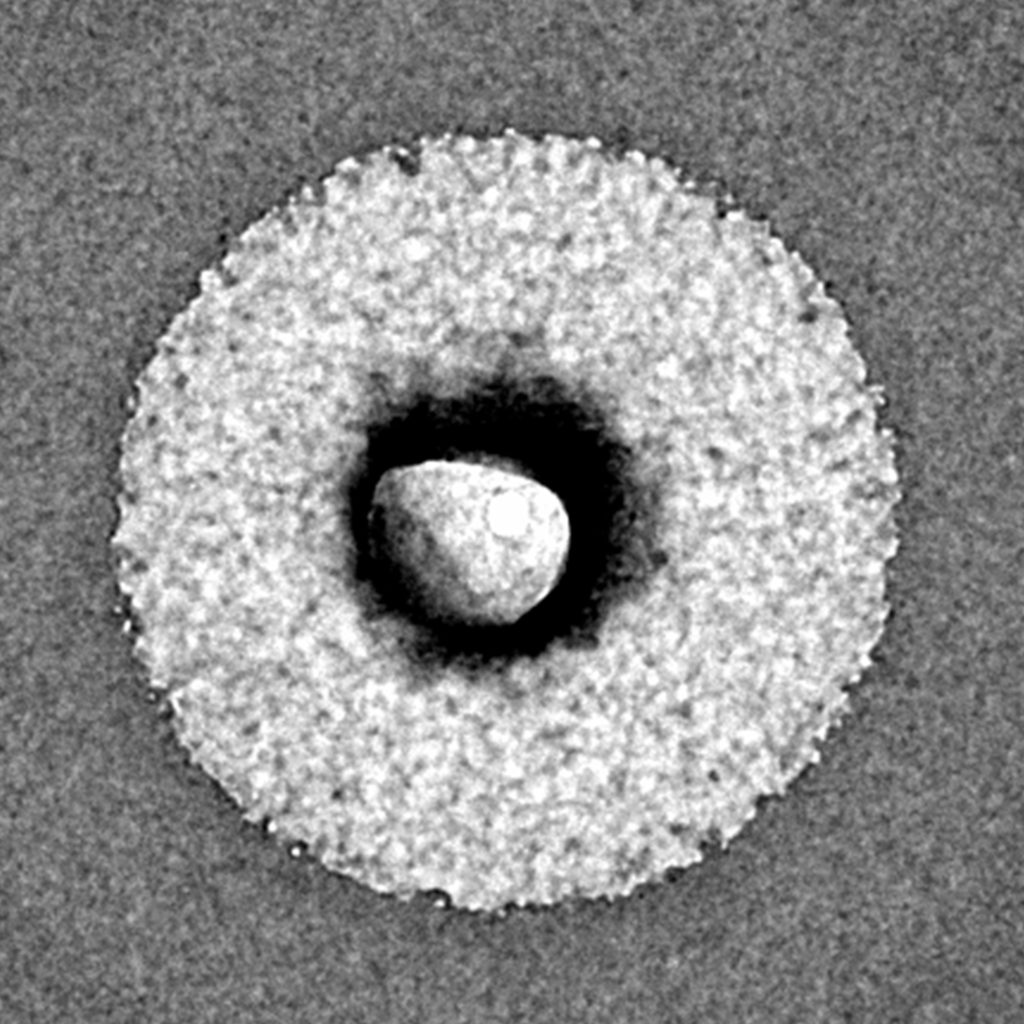
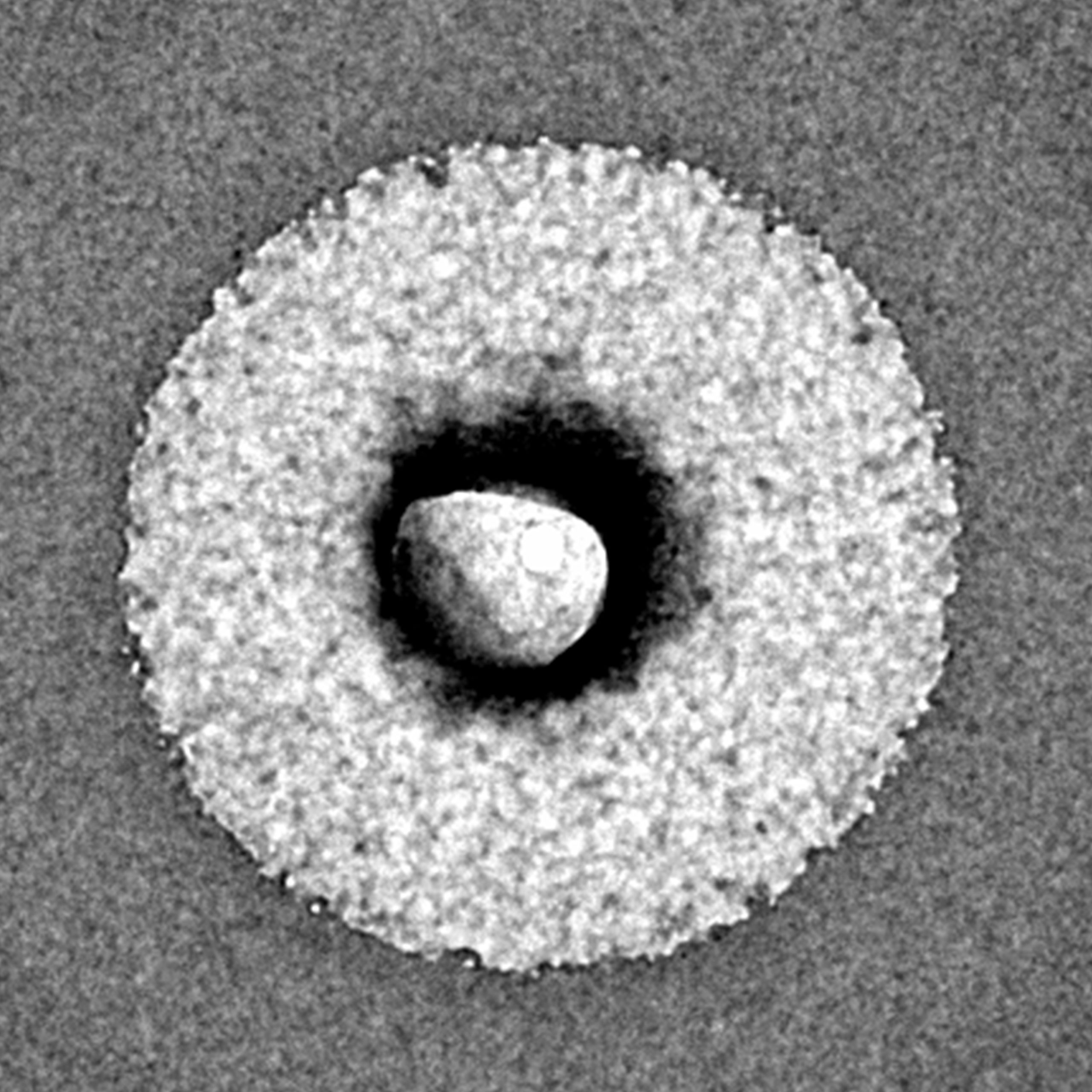
Photo of a single LipoCellTech™ powdered liposome after contact with body fluids. The phospholipids are on the outside and the ingredients in the middle. Magnified 30,000 X with an electron microscope.